
2025-09-16 Posted by TideChem
Conjugating drugs to polymeric carriers can solve the problems of low drug solubility, short half-life, lack of selectivity, and quick removal from the body. Polyethylene glycol (PEG), one of the most widely used polymers for drug conjugation, serves as an ideal polymeric backbone due to its high stability, extended half-life, reduced immunogenicity, ability to control drug release kinetics, and versatile linker functionalization capabilities.
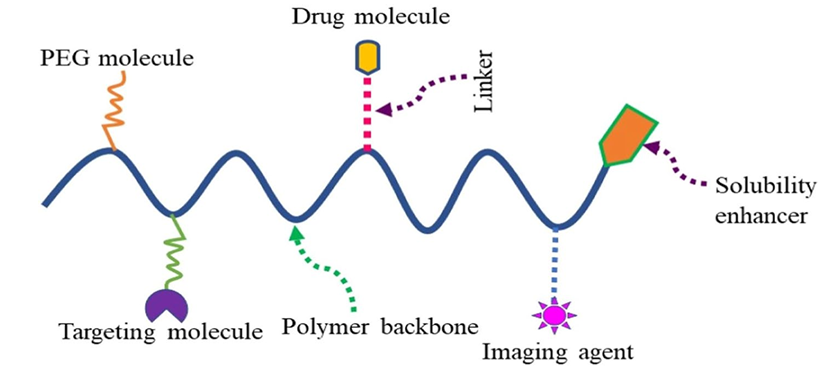
Figure 1. Representation of polymer-drug conjugate for multipurpose use.
Rational molecular design can significantly enhance the therapeutic effectiveness and targeted drug delivery of polymer-drug conjugates based on PEG. When conducting molecular design, the following key aspects must be considered:
(1) The Molecular weight of PEG: The molecular weight of PEG influences the pharmacokinetics and biodistribution of the conjugates.
(2) The architecture of PEG: Branching or multi-arm PEG exhibits higher drug loading capacity and have better targeting efficiency
(3) The compatibility between the drug and the PEG backbone: Considerations such as drug solubility, chemical reactivity, and drug-polymer interaction should guide the selection of appropriate drug candidates for conjugation with PEG.
The effective production of polymer-drug conjugates based on PEG depends on rational linker selection and design. The linker is crucial for regulated drug release, stability, and therapeutic efficacy.
For the polymer-drug combination to remain intact during circulation and to guarantee targeted drug release, the chemical stability of the linker is crucial. Based on their degradation mechanisms within cells, linkers can be classified into two categories: cleavable and non-cleavable. Common types of cleavable linkers include: Enzyme-cleavable peptide linkers, Acid-sensitive hydrazone linkers, Glutathione-sensitive disulfide linkers. In contrast, non-cleavable linkers rely on intracellular lysosomal degradation for drug release and demonstrate superior stability in blood circulation compared to cleavable linkers.
The unique characteristics of the medication and the target site should be taken into account while designing linkers. Selective conjugation with thiol or amino groups present on the medication or targeted ligands is made possible by the insertion of reactive groups, such as maleimide or NHS ester, on the linker.
The linker length and flexibility critically determine the pharmacokinetic properties and therapeutic efficacy of conjugated drugs. Short-chain PEG exhibits relatively rigid characteristics due to constrained molecular conformations, whereas long-chain PEG demonstrates superior structural flexibility and spatial adaptability. The precise modulation of PEG chain length enables performance optimization of drug conjugates to fulfill specific requirements across various application scenarios.
The selection and optimization of conjugation strategies critically determine the therapeutic potency and targeting specificity of drug conjugates. When designing conjugation methods, it is essential to comprehensively evaluate drug release kinetics, stability, and biocompatibility.
(1)Chemical Conjugation
Chemical conjugation is the most commonly used coupling method, where the drug and PEG are functionalized with reactive groups that undergo a specific chemical reaction to form a covalent bond. CDN prodrug containing a dialanine peptide linker was synthesized and conjugated to a thiol-terminated PEG-phospholipid via thiol–maleimide coupling. The resulting CDN-PEG-lipid was designed to facilitate formulation in lipid-based drug carriers, with release of the active STING agonist upon peptidase cleavage in endosomes following cellular uptake.
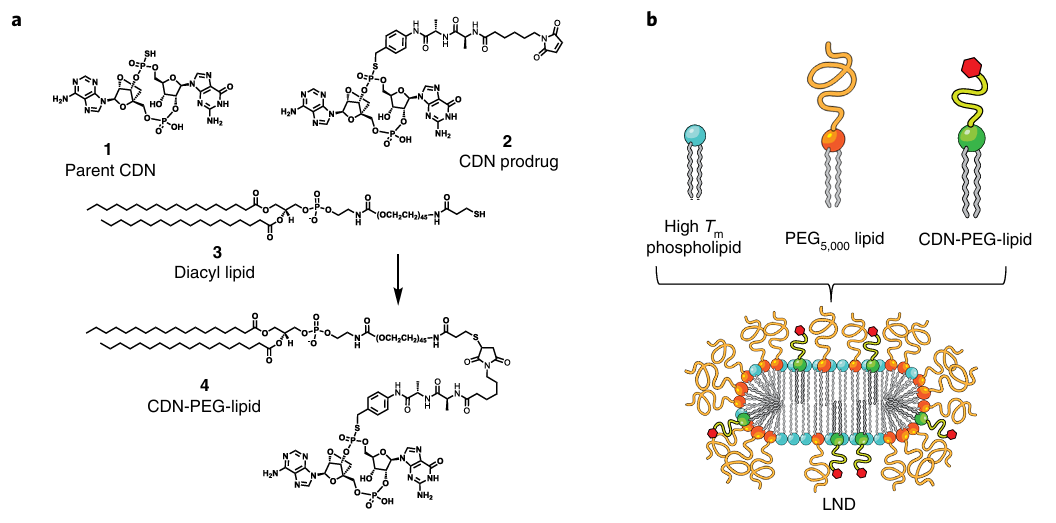
Figure 2. LNDs carrying CDN-PEG-lipid exhibited more efficient penetration of tumours.
(2)Bioorthogonal Conjugation
The advent of bioorthogonal chemistry has brought a revolutionary breakthrough for studying biomolecules in living systems: these chemical reactions selectively occur between abiotic functional groups while remaining inert to biological functionalities. Bioorthogonal reactions must exhibit exquisite selectivity, be high yielding, require simple design and execution, feature non-toxic components, and proceed in physiological media and conditions. Common types of bioorthogonal reactions include: Staudinger ligation, CuAAC, SPAAC, and tetrazine ligation.
For example, PEG lipid (PBA-Lips-Tz) are precisely anchored onto the surface of tumor cells via the orthogonal activator tetrazine (Tz) group. After intravenous injection of PBA-Lips-Tz, the liposomes accumulate in the tumor through PBA-mediated targeting of overexpressed sialic acid on tumor cells. Subsequent perfusion stably incorporates Tz into the membrane, forming a tumor membrane reactor. This strategy provides a solution to the efficacy versus safety dilemma of tumor chemotherapy.
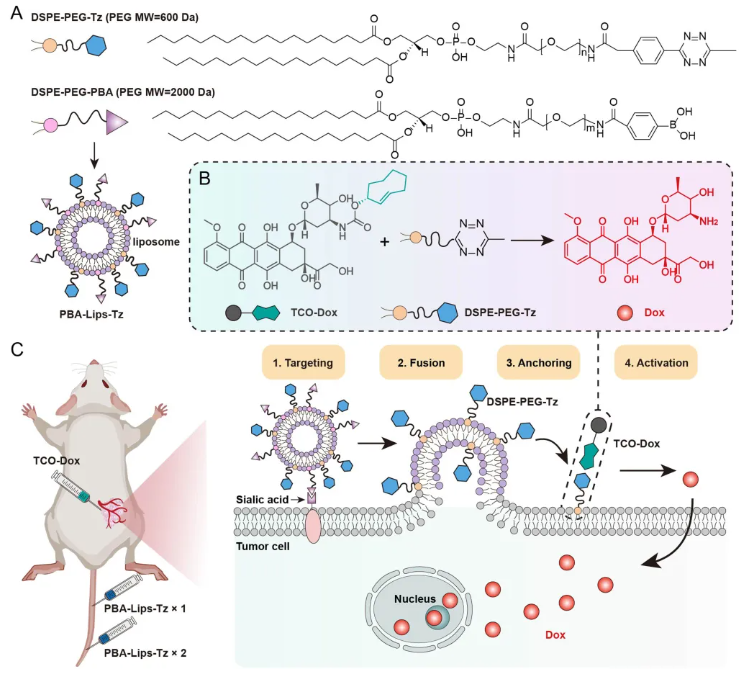
Figure 3. A High-Efficiency Bioorthogonal Tumor-Membrane Reactor.
(3)Enzymatic conjugation
In recent years, Enzymatic conjugation methods have garnered significant attention due to their unique advantages. Compared to traditional chemical conjugation methods, enzymatic conjugation methods enables precise targeting of specific sites on substrates, effectively avoiding byproduct formation. Moreover, its mild reaction conditions (ambient temperature, neutral pH, aqueous environment) perfectly align with the principles of green chemistry. The selection of enzymatic conjugation methods should consider factors such as the stability of the drug, reaction kinetics.
The drug nanocarriers PEG-PPMD and PEG-PCMD were synthesized via enzymatic reactions catalyzed by Candida antarctica lipase B (CALB). Docetaxel (DTX)-loaded PEG-PPMD and PEG-PCMD micelles can be triggered synergistically by acidic endosomal pH and a high intracellular reduction potential to rapidly release the drug for efficient killing of cancer cells. The drug formulations based on PEG-PPMD and PEG-PCMD copolymers exhibited a substantially higher potency than free DTX in inhibiting tumor growth in mice, whereas their therapeutic effects on important organ tissues were minimal.
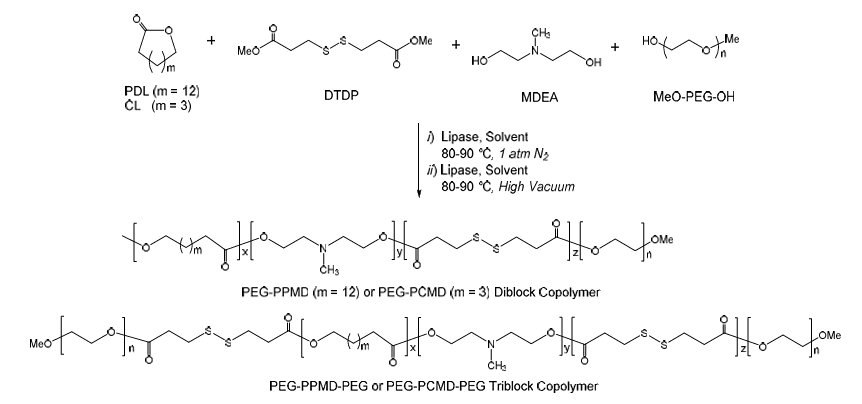
Figure 4. Enzymatic PEG-Poly for Efficient Antitumor Treatment.
Verma, Vinay Sagar et al. “Polyethylene Glycol-Based Polymer-Drug Conjugates: Novel Design and Synthesis Strategies for Enhanced Therapeutic Efficacy and Targeted Drug Delivery.” Applied biochemistry and biotechnology vol. 196,10 (2024): 7325-7361. doi:10.1007/s12010-024-04895-6.
Javia, Ankit et al. “Polymer-drug conjugates: Design principles, emerging synthetic strategies and clinical overview.” International journal of pharmaceutics vol. 623 (2022): 121863. doi:10.1016/j.ijpharm.2022.121863.
Dane, Eric L et al. “STING agonist delivery by tumour-penetrating PEG-lipid nanodiscs primes robust anticancer immunity.” Nature materials vol. 21,6 (2022): 710-720. doi:10.1038/s41563-022-01251-z
Hartung, Kaitlin M, and Ellen M Sletten. “Bioorthogonal chemistry: Bridging chemistry, biology, and medicine.” Chem vol. 9,8 (2023): 2095-2109. doi:10.1016/j.chempr.2023.05.016.
Ma, Yu et al. “A High-Efficiency Bioorthogonal Tumor-Membrane Reactor for In Situ Selective and Sustained Prodrug Activation.” Angewandte Chemie (International ed. in English) vol. 63,10 (2024): e202318372. doi:10.1002/anie.202318372.
Chen, Ya et al. “Enzymatic PEG-Poly(amine-co-disulfide ester) Nanoparticles as pH- and Redox-Responsive Drug Nanocarriers for Efficient Antitumor Treatment.” ACS applied materials & interfaces vol. 9,36 (2017): 30519-30535. doi:10.1021/acsami.7b10148.