
2025-09-24 Posted by TideChem
Polyethylene glycol (PEG) is a polymer with the structure(-CH2CH2O-)n ,which is synthesized normally by ring opening polymerization of ethylene oxide, with molecular weights typically ranging from 200 to 8000 or higher. Due to their excellent water solubility and biocompatibility, PEG linkers are widely used in drug conjugation, biolabeling, and drug delivery systems.
Based on molecular uniformity, there are two classes of PEG linkers, monodispersed and polydispersed. Monodispersed PEG has an exact number of PEG units with a specific single chemical structure with precise molecular weight, whereas polydispersed PEG is a polymer mixture that has an average molecular weight. PEG linkers can be functionalized with a variety of functional groups such as Amine, Mal, NHS, COOH, N3, Biotin, and DBCO, providing versatile chemical handles for further modifications.
(1)Improved water solubility: A dramatic increase in the water solubility of modified molecules.
(2)Reduced Immunogenicity: Increase in immunogenicity due to the shielding of modified molecules from the immune system.
(3)Extended Circulation Time: Protection of modified protein and peptides from digestion by proteolytic enzymes, and effectively increasing their hydrophobic volume and decreasing clearance rates by renal filtration, all of which results in an increase in the serum half-life of modified modified molecules in vivo.
1.PEG Linkers in Antibody Drug Conjugate
Antibody drug conjugates (ADCs) are a novel class of highly potent antitumor drugs, with key components comprising an antibody, a linker, and a payload. The linker serves as a bridge between the antibody and the payload, playing a critical role in the stability and efficacy of ADCs. The linkers of Trodelvy® and Zynlonta® both include PEG units, which improve water solubility and enhance plasma stability to optimize drug treatment efficacy.
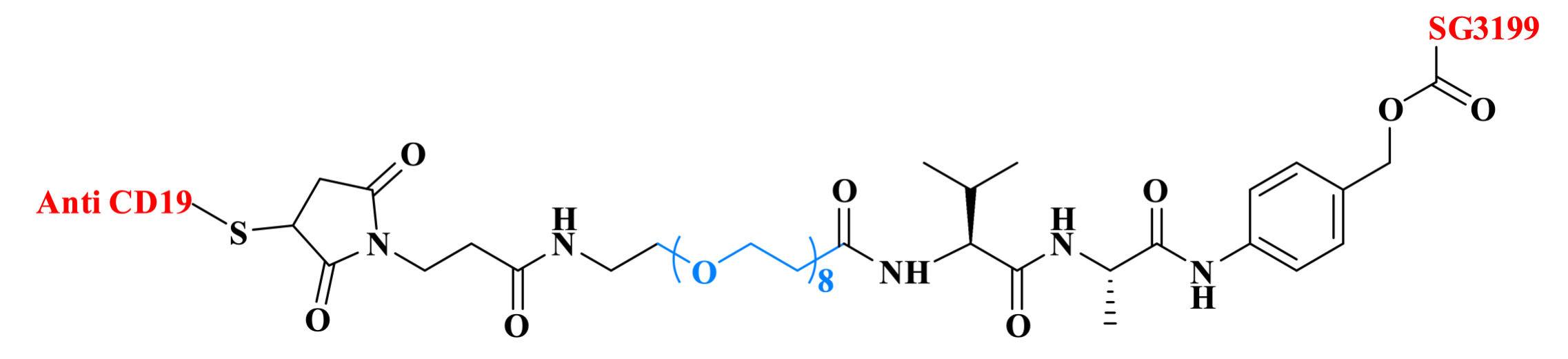
Figure 1. The structure of Zynlonta® contains a PEG8 linker.
2.PEG Linkers in Drug Delivery Systems
Liposomes are phospholipid-based vesicles with aqueous cores enclosed by lipid bilayers, allowing the encapsulation of both hydrophilic and lipophilic drugs. The incorporation of PEG linkers enhances the circulatory half-life of liposomes, reduces immunogenic recognition, and improves therapeutic efficacy in vivo. PEGylated liposomes have the advantages of targeted drug delivery, enhanced drug solubility, controlled and sustained release.
Doxil®, the PEGylated liposomal doxorubicin developed by Sequus Pharmaceuticals, became the first FDA-approved liposomal drug. Its PEGylation technology significantly extends plasma half-life while reducing cardiotoxicity, establishing a milestone in oncology therapeutics.
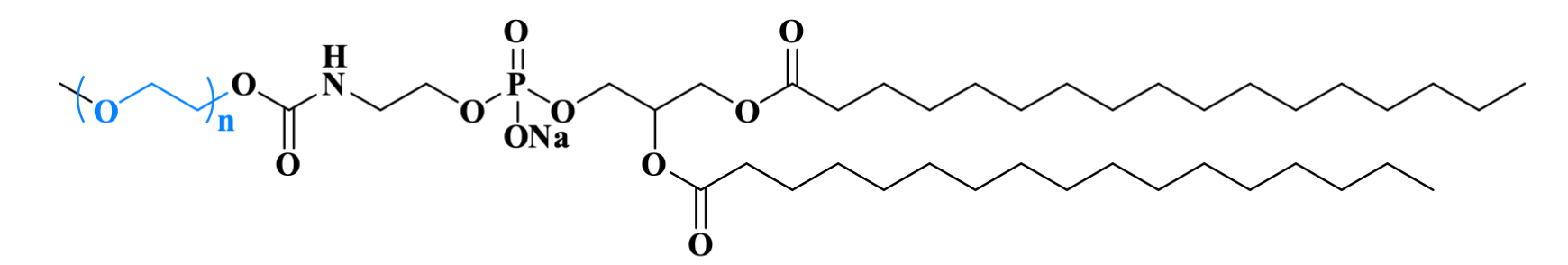
Figure 2. The structure of Doxil® contains DSPE-PEG2000.
3.PEG Linkers in Protein and Peptide Drugs
Protein and peptide drugs are commonly limited by their short plasma half-life and poor oral bioavailability. PEGylated proteins and peptides can increase molecular size to reduce renal clearance and provide steric shielding against proteolytic attack, thereby decreasing compound clearance rates. Clinically successful examples of PEGylated protein drugs include Skytrofa® for growth hormone deficiency and Besremi® for polycythemia vera, both demonstrating optimized pharmacokinetic profiles and enhanced therapeutic efficacy.
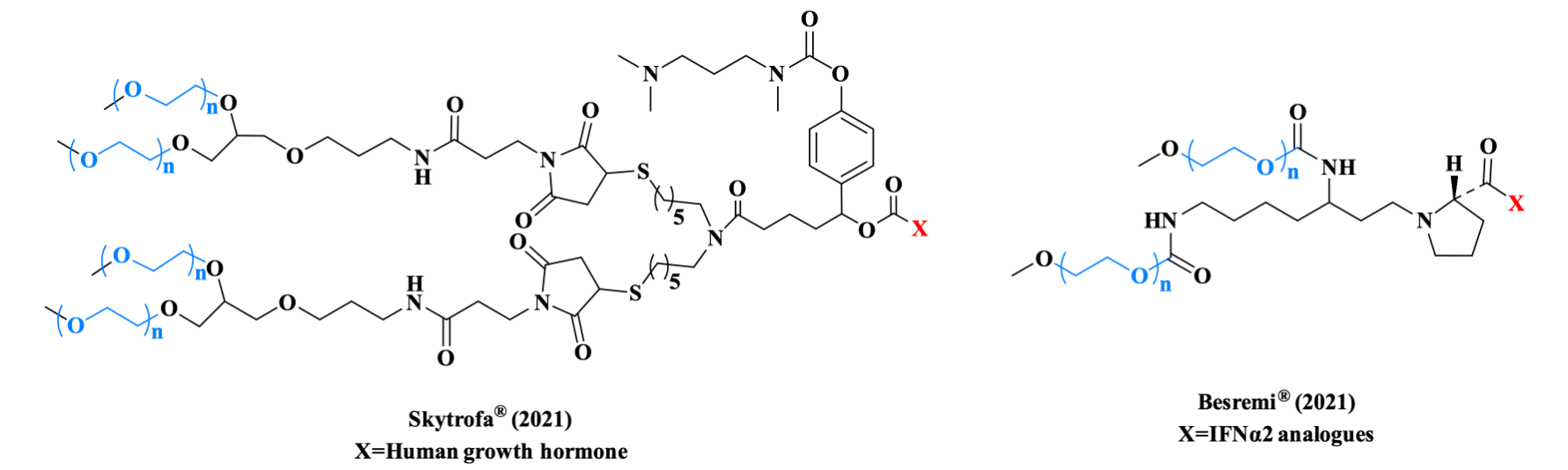
Figure 3. PEG linker used in Skytrofa® (Left) and Besremi® (Right).
4.PEG Linkers in Small Molecule Drugs
Through PEGylation, small molecule drugs can acquire PEG's superior physicochemical properties to form biocompatible complexes, offering an innovative solution for developing long-acting formulations and enabling targeted therapy. A prime example is Movantik®, a PEGylation of small molecule drug approved by the FDA in 2014 for treating opioid-induced constipation.
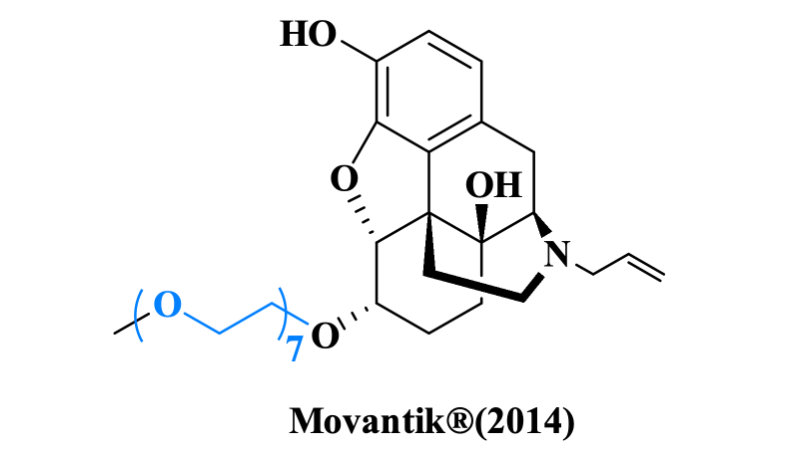
Figure 4. PEG linker used in Movantik®.
Turecek, Peter L et al. “PEGylation of Biopharmaceuticals: A Review of Chemistry and Nonclinical Safety Information of Approved Drugs.” Journal of pharmaceutical sciences vol. 105,2 (2016): 460-475. doi:10.1016/j.xphs.2015.11.015.
Zammarchi, Francesca et al. “ADCT-402, a PBD dimer-containing antibody drug conjugate targeting CD19-expressing malignancies.” Blood vol. 131,10 (2018): 1094-1105. doi:10.1182/blood-2017-10-813493.
Chey, William D et al. “Naloxegol for opioid-induced constipation in patients with noncancer pain.” The New England journal of medicine vol. 370,25 (2014): 2387-96. doi:10.1056/NEJMoa1310246.
Chatelain, Pierre et al. “A Randomized Phase 2 Study of Long-Acting TransCon GH vs Daily GH in Childhood GH Deficiency.” The Journal of clinical endocrinology and metabolism vol. 102,5 (2017): 1673-1682. doi:10.1210/jc.2016-3776.
Quintás-Cardama, Alfonso et al. “Molecular analysis of patients with polycythemia vera or essential thrombocythemia receiving pegylated interferon α-2a.” Blood vol. 122,6 (2013): 893-901. doi:10.1182/blood-2012-07-442012.
Kanemasa, Toshiyuki et al. “Pharmacological Profile of Naldemedine, a Peripherally Acting μ-Opioid Receptor Antagonist: Comparison with Naloxone and Naloxegol.” The Journal of pharmacology and experimental therapeutics vol. 373,3 (2020): 438-444. doi:10.1124/jpet.119.264515.